Patch2022
Todo
Patch2022 will be discontinued. Use Patch instead.
Patch is introduced Walmsley et al.[1]
Module summary
Patch2022 is based on Patch in Walmsley 2015.
Parameters
- Am_ref [\(m^2\)]: float
Reference wall area at \(l_{s} = l_{s,ref}\).
- V_wall [\(m^3\)]: float
Wall volume
- v_max [\(\mu m/s\)]: float
Maximum shortening velocity
- l_se0 [\(\mu m\)]: float
lgth of the series elastic element, i.e. \(l_{s} -l_{si}\) for which stress is zero.
- l_s0 [\(\mu m\)]: float
Reference sarcomere lgth for which at \(A_m (l_{s,ref}) = A_{m,ref}\).
- dl_s_pas [\(\mu m\)]: float
Nonlinear exponent of Titin stress
- Sf_pas [Pa]: float
Linear ECM stress coefficient
- fac_Sf_tit [-]: float
Contribution factor of titen stress multiplied with Sf_act
- k1 [-]: float
Nonlinear exponent ECM stress component
- tr [s]: float
Contraction time constant
- td [s]: float
Relaxation time constant
- time_act [-]: float
Relative contraction duration
- Sf_act [Pa]: float
Linear active stress component
- dt [s]: float
Activation delay relative to intrinsic activation
- C_rest [-]: float
Rest contractility
- l_si0 [\(\mu m\)]: float
Reference lgth for zero-active-stress
- LDAD [s]: float
strain dependend activation duration
- ADO [s]: float
activation duration offset
- LDCC [-]: float
stretch dependend contractility coefficient
- Sf_pasMaxT: float
Maximum ecm stress (adaptation sens variable)
- Sf_pasActT: float
Active weighted passive stress (adaptation sens variable)
- FacSf_actT: float
Active stress (adaptation sens variable)
- LsPasActT: float
Weighted sarcomere lgth average (adaptation sens variable)
- adapt_gamma: bool
Adaptation constant
Signals
Signals are arrays. Each point in the array represents a point in time with step-size controlled by the solver.
- l_s [\(\mu m\)]: array
Sarcomere lgth
- l_si [\(\mu m\)]: array
State variable: Intrinsic sarcomere lgth
- LsiDot [\(\mu m/s\)]: array
State variable: Intrinsic sarcomere lgth time-derivative
- C [-]: array
State variable: contraction curve
- C_dot [1/s]: array
State variable: contraction time-derivative
- Am [m:sup:2]: array
Patch mid-wall area
- Am0 [m:sup:2]: array
Patch mid-wall zero-stress area
- Ef [-]: array
Natural strain
- T [Nm]: array
Mid-wall tension
- dA_dT [m / N]: array
Area-tension derivative
- Sf [Pa]: array
Total fibre stress at mid-wall
- Sf_pasT [Pa]: array
Total passive stress at mid-wall
- SfEcm [Pa]: array
Total ECM stress at mid-wall
- dSf_dEf [Pa]: array
Total stiffness coefficient
- dSf_pas_dEf [Pa]: array
Total passive stiffness coefficient
- SfEcmMax: array
Adaptation: Maximum ECM stress
- Sf_actMax: array
Adaptation: maximum active stress
- Sf_pasAct: array
Adaptation: active-weighted passive stress
- LsPasAct: array
Adaptation: active-weigthed sarcomere lgth
Physiological Background
In a normal adult, the heart has four chambers, the left and right atrium and the left and right ventricle. Two valves connect the atria with the ventricles. The tricuspid valve is positioned between the right atrium and the right ventricle, and the mitral valve between the left atrium and the left ventricle. Besides these atrio-ventricular inlet valves, each ventricle has a ventricular-arterial outlet valve connecting them to the systemic and pulmonary circulation. The pulmonary valve lies between the right ventricle and the pulmonary artery, and the aortic valve lies between the left ventricle and the aorta. Wall strain determines the wall tension resulting from the passive and active material behavior of the myocardial tissue. Wall tension together with geometry determine the cavity pressure.
The pressure in a heart chamber is determined by the cavity volume. The cavity volume determines the myofibers strain. Myofiber stress arises from myofiber strain. The myofibers have a well-documented helical arrangement in the ventricles (Streeter Jr et al.[2]), and have a considerably more complex arrangement in the atria (Ho and Sanchez-Quintana[3]). Besides, the myofibers are loosely arranged into sheet-like structures. Myofiber stress is a summation of active stress, generated by the sarcomere, and passive stress resulting from stretch of elastic structures, primarily attributed to the extracellular matrix (ECM). The stress-strain relationship of the myocardial tissue is discussed in more detail in the documentation for the sarcomere module. The atria may be considered as simple cavities, encapsulated each by a relatively thin contractile elastic wall. In the CircAdapt model (Arts et al.[4], Lumens et al.[5]), each atrium is simulated by a Chamber-module. The ventricular unit is more complicated, because the right and left ventricle interact by their shared wall, the septum (Lumens et al.[5], Lumens et al.[6]). In the CircAdapt model, the composition of the left and right ventricle is simulated by a TriSegmodule. Mechanical interaction of the atrial walls with each other and with the ventricular walls probably occurs in the human heart, but is neglected because these interactions are relatively insignificant (Goldstein et al.[7], Henein et al.[8]).
In many examples of cardiac pathology, mechanical properties are inhomogeneously distributed over the various walls. To simulate such inhomogeneities in CircAdapt, the wall has been subdivided in a number of patches, each having specific mechanical properties. A patch is modeled by a Patch2022-module, allowing to have specific timing of activation, specific contractile properties and specific passive elastic properties. The Wall2022 links the Patch2022 mechanics to global hemodynamics.
Sarcomere Mechanics
The myocardial tissue module Patch takes as its inputs mid-wall segment tension \(T_m\), mid-wall curvature \(C_m\), and time \(t\), and outputs fibre stiffness \(dA_m/dT\) and zero-tension reference area \(A_m0\). Each initialized module object consists of two state variables, namely the contractile element length \(l_{si}\) and the contractility curve \(C\). Myocardial tissue is deformable (soft) and incompressible. Sarcomere mechanics are assumed homogeneous in a single segment, meaning one single sarcomere represents the entire segment. According to the one-fiber model (Lumens et al.[5]), tension is related to wall stress by
Therefore, \(dA_m/dT\) is given by
and \(A_{m,0}\) is given by
Hill element
A sarcomere is modelled as a three-element Hill contraction model (Fung[9]), in which the elastic element (with length \(l_{se}\)) and contractile element (with length \(l_si\)) in series are in parallel with an elastic element (\(l_s=l_{se}+l_{si}\)), which is calculated as
Fiber strain is derived from mid-wall area \(A_m\), wall curvature \(C_m\), and wall volume \(V_w\) (Lumens et al.[5]).
in which \(l_{s,ref}=2\) is the reference length of the sarcomere at reference wall area \(A_{m,ref}\). The intrinsic sarcomere length is a delayed image of the sarcomere length and is given by the following differential equation
in which v_max is the unloaded sarcomere shortening rate and \(l_{se,iso}\) the length of series elastic element. The total sarcomere length will decrease at a rate proportional to the length of the series elastic element when the cell is unloaded [deTOMBE]. Total fibre stress is the sum of two stress components, an active stress generated by sarcomere contraction, and a passive stress arising from structures such as the ECM and titin. Both passive and active stress depend upon the length of the sarcomere.
Active behavior
The active stress depends also on time through a contractility parameter \(C\). Contractility is a phenomenological quantity representing the density of cross-bridges formed in the sarcomere. There is a resting value of contractility, which may be non-zero. This can represent residual cross-bridge formation during diastole. Contractility increases when the tissue is activated. Activation is smooth and has a rise and decay phase with different time constants. The rate of change of contractility increases with sarcomere length (Kentish et al.[10]). Active stress increases with contractility, contractile element length and series elastic element length. Based on these assumptions, the contractility curve \(C\) is implemented as a state-variable and given by
with
This function uses the function \(f_{rise}\) to modulate the rise of the contractility and \(f_{decay}\) to modulate the decay of contractility.
The first is given by:
Crossbridge formation function \(C_L (l_{si})\)
Decay function \(g(X)\)
From the contractility curve, the total active stress is calculated using
(Source code, png, hires.png, pdf)
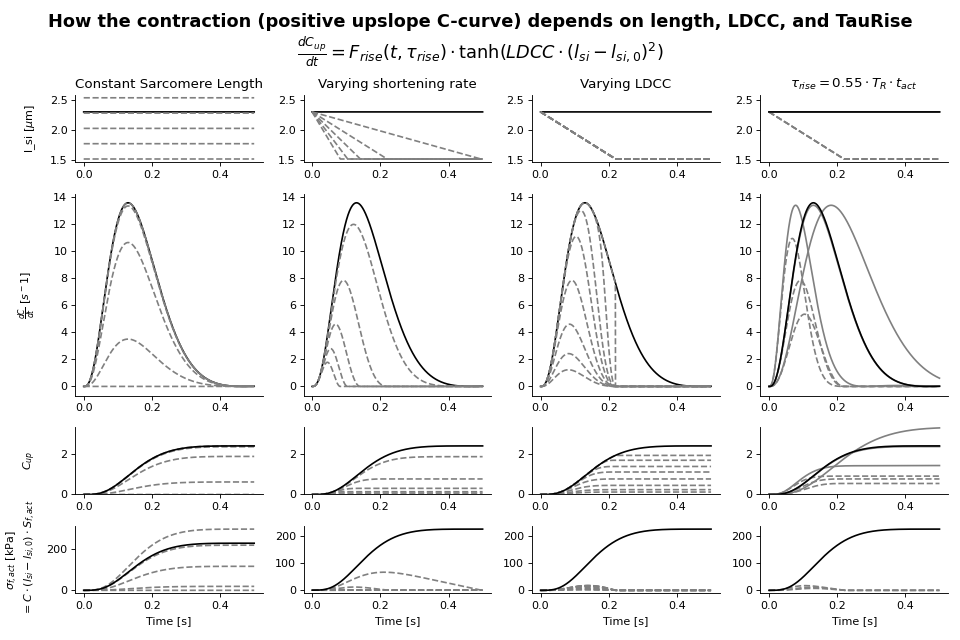
Passive behavior
(Source code, png, hires.png, pdf)
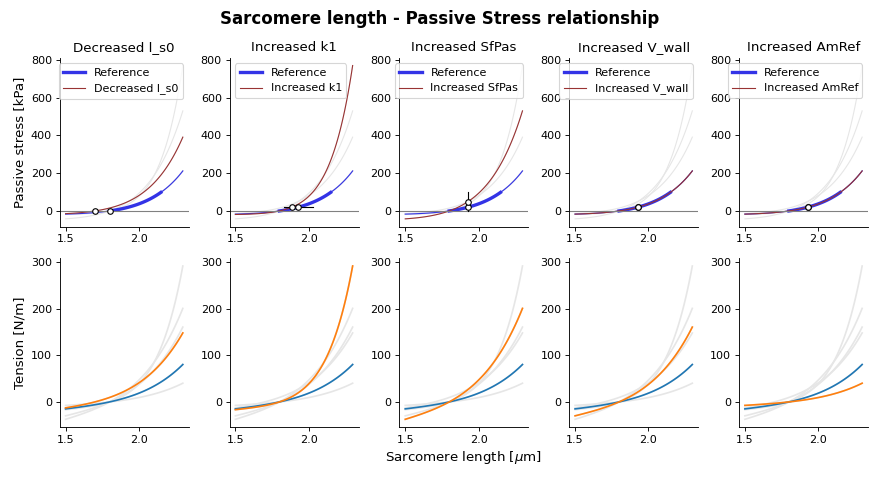
Fig. 17 Passive stress-strain relation as function of sarcomere length
(Source code, png, hires.png, pdf)
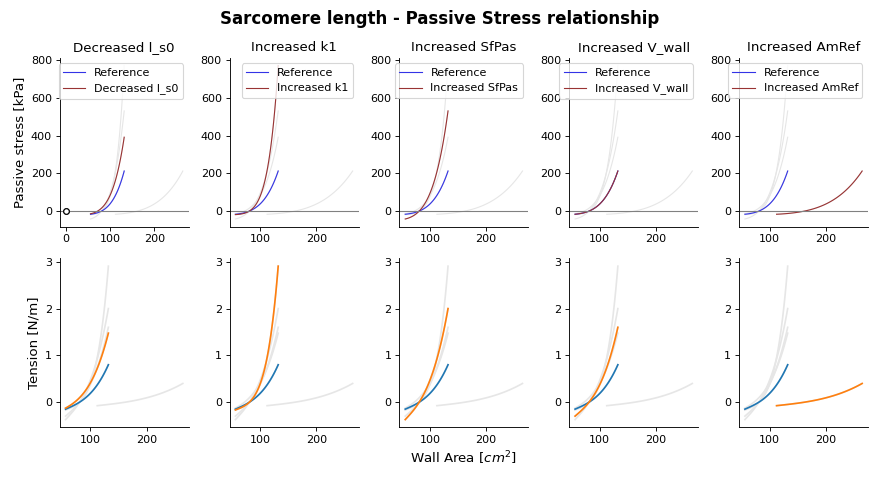
Fig. 19 Passive stress-strain relation as function of wall area
Assumptions
Not Included in this Version of the Model:
Force-frequency relationship (force of contraction increases with heart rate).
Electrophysiology model, only imposed activation times.
Biophysics / energetics of calcium transient, cross-bridge formation, etc - currently, this is modelled phenomenologically and availability of ATP is assumed to be infinite.
Sympathetic / para-sympathetic stimulation of cardiomyocytes.
References
Patch2022 is based on Patch in Walmsley 2015. |